Protein histidine methylation
The enzyme is called METTL9, which stands for Methyltransferase like 9, and its function was unknown until now. The scientists were now able to identify it as a protein histidine methyltransferase - an enzyme that transfers methyl groups to histidine, one of the amino acids that form proteins. Like other chemical modifications on the proteins of the cell, methylation has an important role to play. It regulates and optimizes protein activity in the control of cellular functions.
Protein histidine methylation, which was already described a good 50 years ago, has the potential to regulate many molecular interactions and cellular processes. Depending on where the methyl group is transferred to the histidine molecule, histidine can occur in the forms of the two isomers 1- or 3-methylhistidine. However, the biochemical process has not yet been researched further. With their work, the researchers were now able to show that METTL9 generates a large part of 1-methylhistidine in the cells.
HisxHis motif as a recognition sequence
Erkennungssequenz HisxHis-Motiv
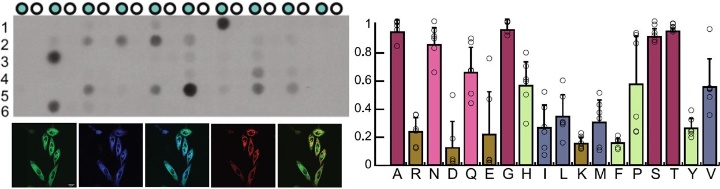
Furthermore, the Stuttgart researchers were able to demonstrate that METTL9-mediated methylation requires a HisxHis motif as a recognition sequence. This means that METTL9 becomes active, and transfers a methyl group to a histidine molecule, only at those sites of the protein where there are two consecutive histidines (His). Between these, there should preferably be a small amino acid residue (x).
For this detective work, the scientists used peptide array technology. Using this method, very small amounts of protein fixed on a carrier material enabled the scientists to quickly and economically test a large number of different protein sequences with respect to their methylation by METTL9.
Push for the cellular power plant
The researchers also found out what effect the METTL9-mediated protein modification has on metabolic processes in the cell. What is particularly interesting: The enzymatic activity of METTL9 is important for the optimal functioning of complex I-mediated mitochondrial respiration. This is a part of those metabolic processes in the cell that are responsible for energy production and result in the synthesis of ATP as an energy carrier. In the mitochondria, which are the “power plants” of the cell, cellular respiration is stimulated by methylation of a mitochondrial protein. As a result, the cellular power plant is more efficient and produces more ATP. This observation suggests that future research work will bring further exciting insights into the biological role of METTL9. New findings may then be able to explain why genetic defects regarding METTL9 are correlated with deafness and inflammatory bowel diseases, and could thus identify new treatment approaches for these diseases.
Original publication:
Pål Ø. Falnes et al. The methyltransferase METTL9 mediates pervasive 1-methylhistidine modification in mammalian proteomes, Nature Communications volume 12, Article number: 891 (2021) https://www.nature.com/articles/s41467-020-20670-7
Contact:
Prof. Dr. Albert Jeltsch, Universität Stuttgart, Institut für Biochemie und Technische Biochemie (IBTB), Tel.: 0711/ 685-64390, E-Mail